Introduction
The periodic table is a systematic arrangement of chemical elements that forms the foundation of chemistry. It not only showcases elements’ atomic structure but also illustrates their relationships with one another. Understanding the periodic table is crucial for students, educators, and scientists alike as it allows for predictions of chemical behaviour and encourages a deeper comprehension of elemental properties.
Structure of the Periodic Table
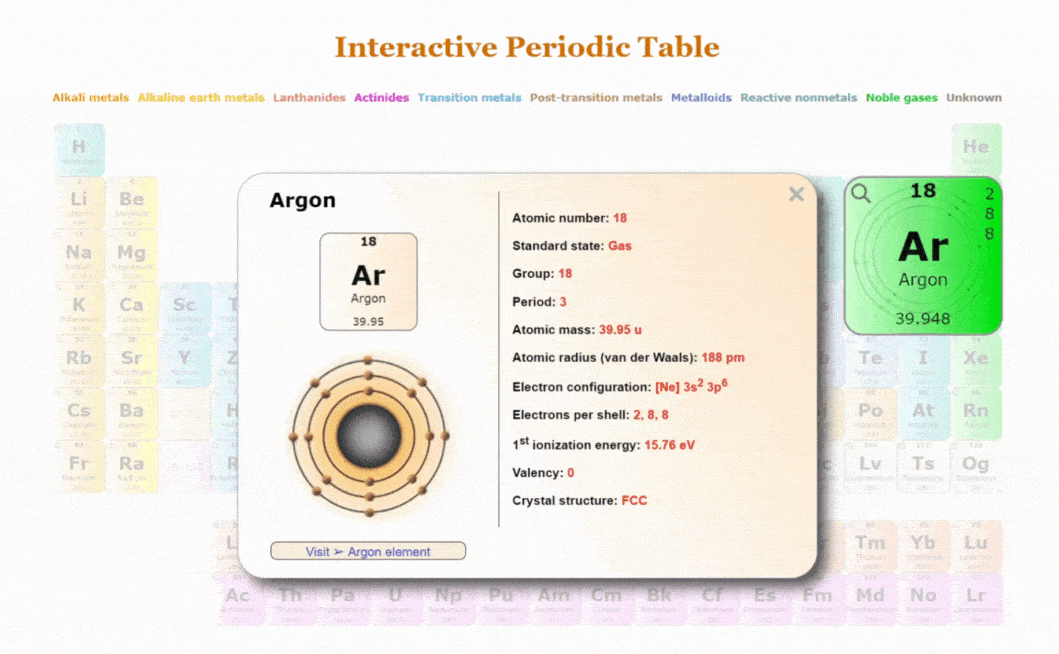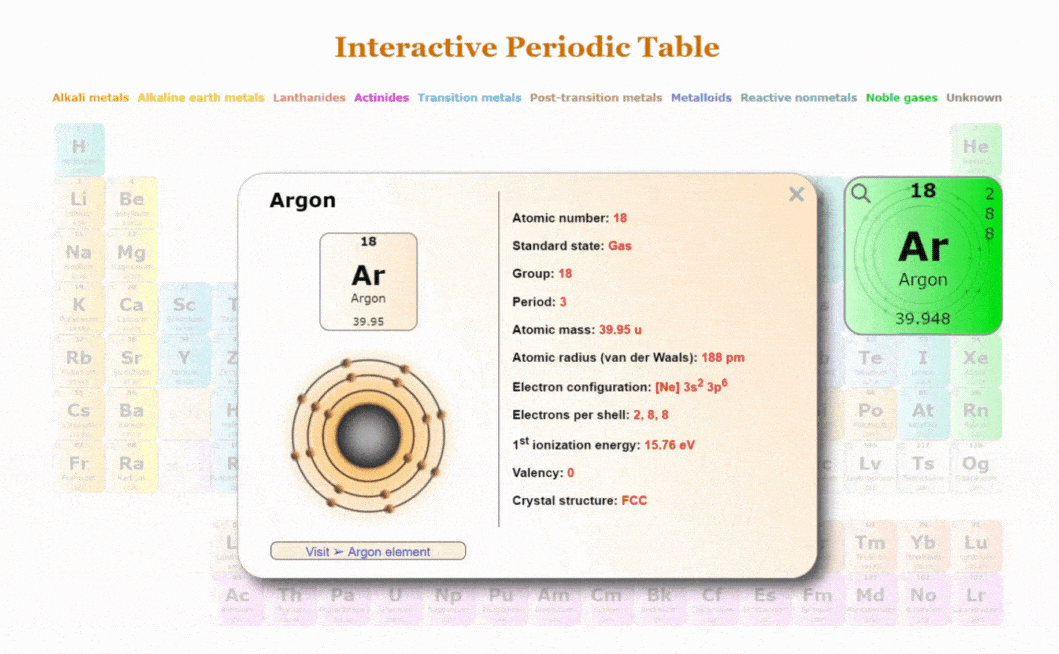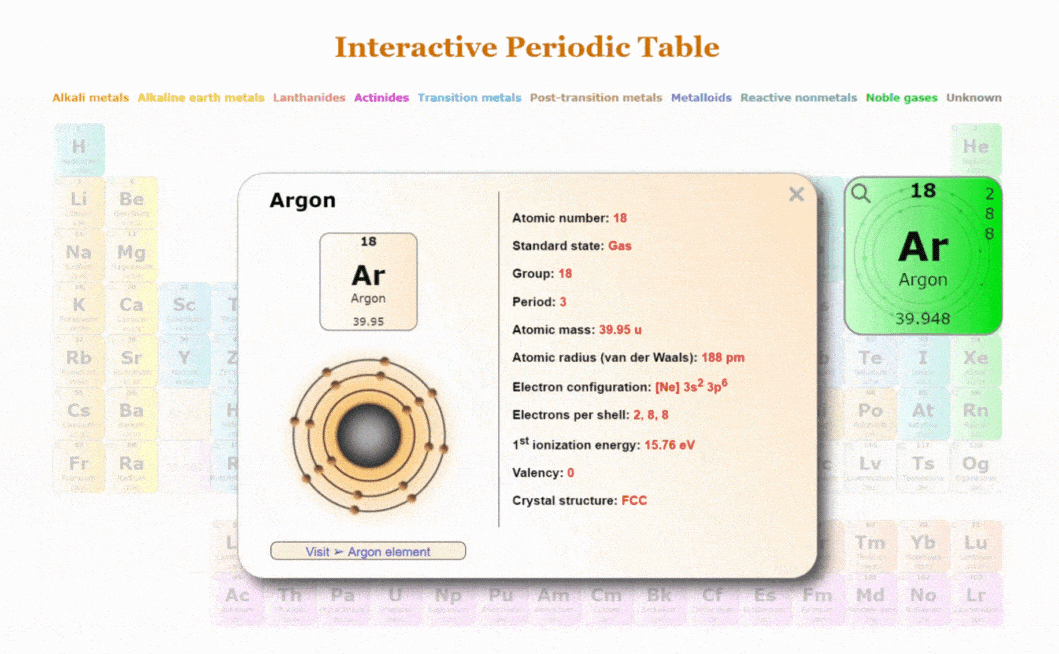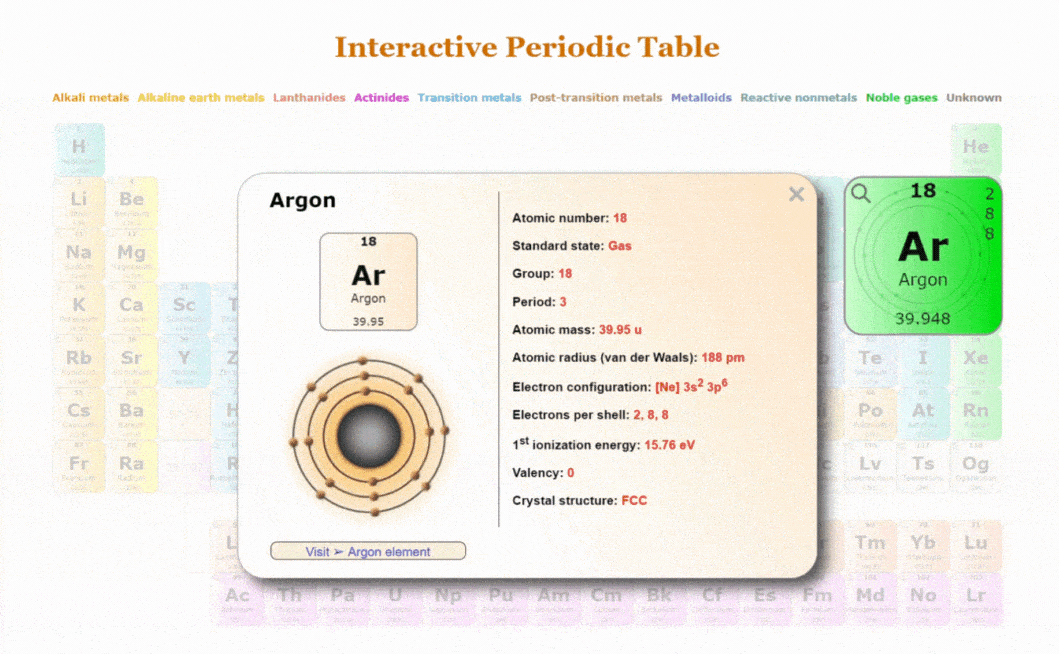
The periodic table is divided into rows called periods and columns known as groups or families. Elements within the same group exhibit similar chemical properties due to their valence electron configurations. For example, alkali metals in Group 1 are highly reactive with water, while noble gases in Group 18 are inert due to their complete electron shells.
The modern periodic table is based on the atomic number, which represents the number of protons in an atom’s nucleus. This arrangement gives rise to trends in the table, such as electronegativity, atomic radius, and ionisation energy, which all vary predictably across periods and groups. The table consists of primary blocks: s-block, p-block, d-block, and f-block elements, each providing distinct characteristics.
Recent Developments and Future of the Periodic Table
In recent years, scientists have focused on the discovery and synthesis of new elements, most notably the elements with atomic numbers 113 (Nihonium), 114 (Flerovium), 115 (Moscovium), 116 (Livermorium), 117 (Tennessine), and 118 (Oganesson). These elements have underscored the periodic table’s adaptability as science progresses, continuously expanding our understanding of matter.
As we advance into the future, research in fields like quantum chemistry and materials science may prompt revisions or enhancements in how the periodic table is utilised or interpreted. The emphasis on sustainability and green chemistry is likely to influence elemental applications, motivating a reevaluation of how certain elements are portrayed and utilised in industry and research.
Conclusion
The periodic table remains a vital tool for scientific inquiry and education. Its significance lies not only in presenting elements in an organised manner but also in facilitating connections that foster new discoveries and innovations. As additional elements are synthesised and as applications evolve, the periodic table will continue to serve as a beacon for future discoveries in the realm of science and technology.